Features and Benifits
The Power of Iodine
AIS Brochure
AIS Iodine Dosing System
AIS Promo |
The Power of Iodine
Technology and operating methods
Iodine, a powerful disinfectant for over 100 years, is further enhanced by AIS®’s technology to maximise its superior performance. As a result, the AIS® system uses very low dosages of iodine (1ppm to 30ppm) to achieve outstanding kill rates on fungi, bacteria and viruses.
Iodine - a dark, dense, crystalline solid (4.96 g/ml) at room temperature - slowly dissolves in water to form a concentrated solution, in equilibrium with its crystallised form, of about 250 ppm (0.25g/litre). The AIS® system uses specially manufactured iodine, BioMaxA® / AIS Iodine, which dissolves up to four times faster than regular iodine.
Why iodine kills micro-organisms: Iodine dissolved in water is a potent broad-spectrum biocide even at low concentrations (1–30ppm). Iodine (I2) accepts an electron (e-) from the molecule it is reacting with in a process called oxidation and turns the iodine molecule into the non biocidal iodide (I-) ion:

When in contact with micro-organisms such as bacteria, viruses, fungi and protozoa, iodine is able to rapidly penetrate their cell walls and oxidise a number of critical components within the cell. The combined effect of these oxidative reactions is cell death.
Toxic disinfectant replacement: Extensive and ongoing laboratory testing has demonstrated outstanding results in fungal kill rates on a wide range of fruit and vegetables (efficacy data available on request). The unique opportunity to replace certain toxic fungicides with a highly effective biocide in an environmentally clean process that will remove all by-products is highly significant.
Iodine vs chlorine – why AIS® delivers the most powerful biocide:
Active killing agent: Chlorine in solution converts to two compounds: Hypochlorous Acid (HOCl) and Hypochlorite (OCl-). HOCl is biocidal and will remain in solution only at pH above 6.5, (Below this pH, HOCl breaks down and will gas off quickly out of solution.)
In contrast, iodine breaks down into four principal compounds: Iodine (I2), Hypoiodious Acid (HIO), Triiodide (I3-) and Iodate (IO3-). Both I2 and HIO are strong biocidal agents. I3- and IO3- are only present in very low concentrations and only significant at very high pH; greater than 8.5.
Effect of pH: To maximise the concentration of the required HOCl chlorine compound, the wash water must be maintained within a narrow pH range (6.5 to 7.5). However, the natural chemical consequence of adding chlorine to water is to alter the pH level, dramatically affecting biocidal action. At a pH of 8.0, the active HOCl has dropped to only 25% of its level at pH 6.5. This level is also less than 30% as effective as iodine at the same pH. In addition, chlorine reacts three times faster than iodine with proteins. The effect of this reactivity is to substantially and quickly reduce the effective biocidal action of chlorine in solutions with a high organic load.
The active iodine compounds, I2 and HIO, remain effective at much higher concentrations over a much wider pH range (pH 3.0 to pH 8.5).
The AIS®system and its unique anion exchange resin: The AIS® system optional resin model continuously removes disinfection by-products in an environmentally clean process by running the wash water through its proprietary anion exchange resin. At the same time, diatomic iodine (I2) is being added to the wash water throughout the day. By using the AIS® system in this way, species of iodine that dominate are diatomic iodine and hypoiodious acid – the most powerful biocidal species of the iodine family.
Measuring iodine: AIS®’s purpose-built, iodine-specific electrodes convert the millivolt (mV) reading received from the iodine in solution directly to a ppm reading. Due to the unique properties of iodine, the AIS® system can monitor, record, control and adjust the active iodine in solution in real time and at ppm rates from 0.5ppm to 100ppm. This ability to so accurately control the active biocidal ingredient is in stark contrast to chlorine which cannot be accurately controlled automatically at any concentration above 8ppm.
Trihalomethanes: Governments and environmental agencies globally regulate the maximum level of trihalomethanes (THMs) - cancer-causing chlorine by-products - allowed in wastewater. They are now restricting the release of chlorinated wash water into the environment to prevent further contamination. Treatment of chlorinated water to remove THMs is an expensive exercise.
By comparison, iodine as used in the AIS® system does not produce undesirable by-products or THMs either on the produce or in the wash water.
Iodine and minimising corrosion: The corrosion effect of chlorine is dramatically greater than the iodine delivered in the AIS® system for the following principal reasons:
Due to the difference in both oxidation potential and atomic weight between chlorine and iodine, the corrosion effect of chlorine is more than seven times that of iodine;
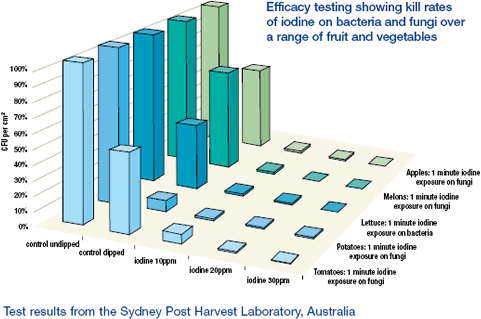
As shown above, the dosages of iodine necessary to be as effective or more effective than chlorine are between five to ten times less than chlorine. So, not only is the iodine significantly less corrosive, but the dosages present are also significantly less. Once installed, the AIS® system will result in significantly longer equipment life, leading to substantial capital equipment cost savings.
|